Newsmatro

Washington, D.C. – The Food and Drug Administration (FDA) has granted authorization for updated COVID-19 vaccines designed to target more recent viral variants. This decision comes after an advisory committee recommended the move earlier this summer.
A separate advisory committee is scheduled to meet on Tuesday to provide recommendations on the utilization of these vaccines.
COVID-19 infections have been on the rise since early July, as reported by the Centers for Disease Control and Prevention (CDC). Hospitalizations have increased by nearly 16%, with deaths rising by almost 17% in the week ending Thursday compared to the preceding week. Despite these increases, the totals remain significantly lower than previous peaks.
There remains ongoing debate within the medical community regarding the necessity of booster shots for everyone. Experts agree that individuals with multiple health issues or weakened immune systems due to age, illness, or medications should receive booster shots at least once a year, possibly more frequently.
Dr. Paul Offit, Director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, emphasized that those who are immune-compromised or suffer from conditions like obesity, diabetes, chronic heart, liver, kidney, or neurologic diseases would benefit from boosters. However, he believes that young, healthy individuals may not require annual shots, as they are at lower risk for severe COVID-19 and already possess some level of protection from previous vaccinations and infections.
Dr. Jesse Goodman, a professor of medicine and infectious diseases at Georgetown University, concurred, noting that the virus responsible for COVID-19 has not mutated as extensively as the influenza virus that necessitates annual flu shots. Consequently, most healthy individuals retain some level of protection. Both Offit and Goodman spoke on Monday as part of the COVID-19 Vaccine Analysis Team, an informal group of vaccine experts.
While the FDA’s actions on Monday pertained specifically to vaccines produced by Moderna and Pfizer-BioNTech, both vaccines will be updated to target the omicron variant XBB.1.5, which dominated during spring and early summer. These vaccines are expected to provide protection against other variants as well.
The FDA’s actions on Monday encompassed the following:
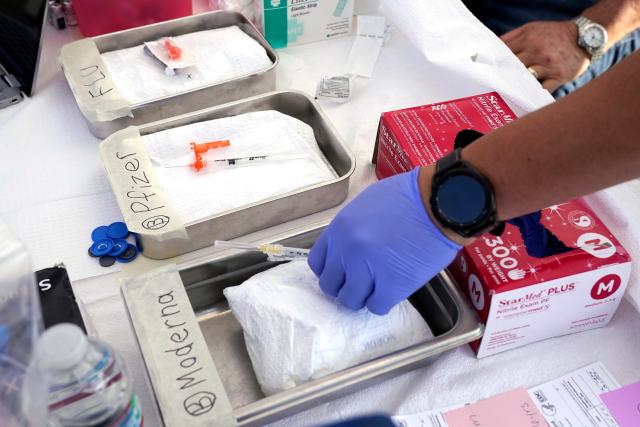
The Advisory Committee on Immunization Practices will convene on Tuesday from 10 a.m. to 4 p.m. EDT to deliberate on these vaccines and their recommended usage. The committee could recommend these vaccines for individuals of all ages or only for high-risk groups.
It’s worth noting that, unlike previous stages of the pandemic, the government will no longer cover the cost of all COVID-19 vaccinations. While individuals with health insurance should have coverage for the shot, those without insurance may need to pay out of pocket, with a cost ranging from $110 to $130 per shot.
Regarding the Novavax vaccine, a third vaccine option, which differs from mRNA-based vaccines and is protein-based, is ready for delivery but was not included in the FDA’s recent actions. Novavax is set to present updated data on its vaccine during the committee meeting. The FDA is considering emergency authorization for its use in individuals aged 12 and above.
John Jacobs, President and CEO of Novavax, stated that the company is prepared for the commercial delivery of their updated non-mRNA COVID vaccine this fall and is working closely with the FDA on its Emergency Use Authorization application.
This health and patient safety coverage is made possible in part by a grant from the Masimo Foundation for Ethics, Innovation, and Competition in Healthcare. The Masimo Foundation does not provide editorial input.