Newsmatro
Recently, a study has uncovered a concerning connection between AstraZeneca’s Covishield vaccine and a rare, potentially fatal blood clotting disorder known as vaccine-induced immune thrombocytopenia and thrombosis (VITT).
This revelation comes in the wake of AstraZeneca’s decision to globally withdraw its coronavirus vaccine due to dwindling sales and the availability of alternative options in the market.
Conducted by researchers from Flinders University, Australia, the study, published in the New England Journal of Medicine, sheds light on the emergence of VITT in 2021, particularly following the widespread administration of the Oxford-AstraZeneca vaccine, which relies on adenovirus vectors.
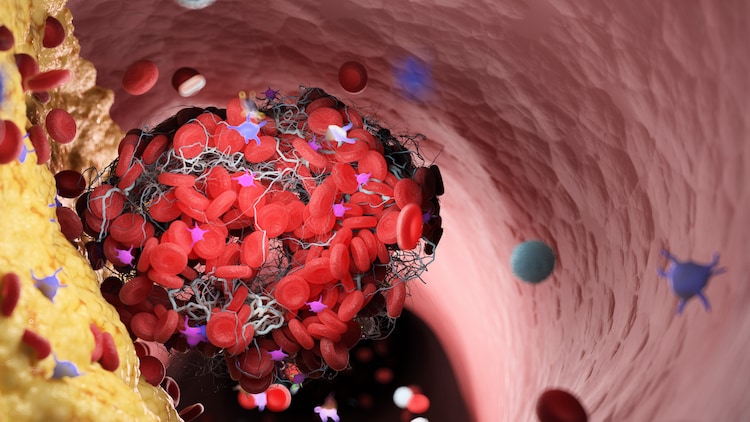
VITT, as elucidated by the study, is triggered by a harmful blood autoantibody that targets a protein called platelet factor 4 (PF4).
Notably, previous research in 2023 had identified a similar disorder associated with natural adenovirus infections, such as the common cold, also involving the PF4 antibody.
Autoantibodies, a type of antibody produced by the immune system, erroneously target and attack the body’s own tissues, leading to autoimmune diseases where healthy cells and tissues are harmed.
This autoimmune response often results in the formation of blood clots in unusual locations like the brain or abdomen, accompanied by elevated levels of a substance called D-dimer in the blood.
Dr. Jing Jing Wang and Professor Tom Gordon from Flinders University, who had previously identified a genetic risk factor related to the PF4 antibody in 2022, collaborated with international researchers to uncover that PF4 antibodies in both vaccine-related VITT and natural adenovirus infections exhibit identical molecular signatures.
The study’s innovative methodology, developed at Flinders University, highlights a common factor in viruses and vaccines that triggers these harmful antibodies.
Moreover, it suggests that the mechanisms underlying antibody production in these disorders are remarkably similar and share comparable genetic risk factors.
Professor Gordon emphasized the significant clinical implications of these findings, noting that lessons learned from VITT could inform the management of rare blood clotting cases following natural adenovirus infections and contribute to enhancing vaccine safety.
For individuals concerned about VITT, the American Society of Hematology indicates that symptoms typically manifest within 4 to 42 days after receiving the Covid vaccine.
However, it’s essential to remain vigilant and seek medical attention if experiencing severe headache, visual changes, abdominal pain, nausea and vomiting, back pain, shortness of breath, leg pain or swelling, or easy bruising or bleeding.